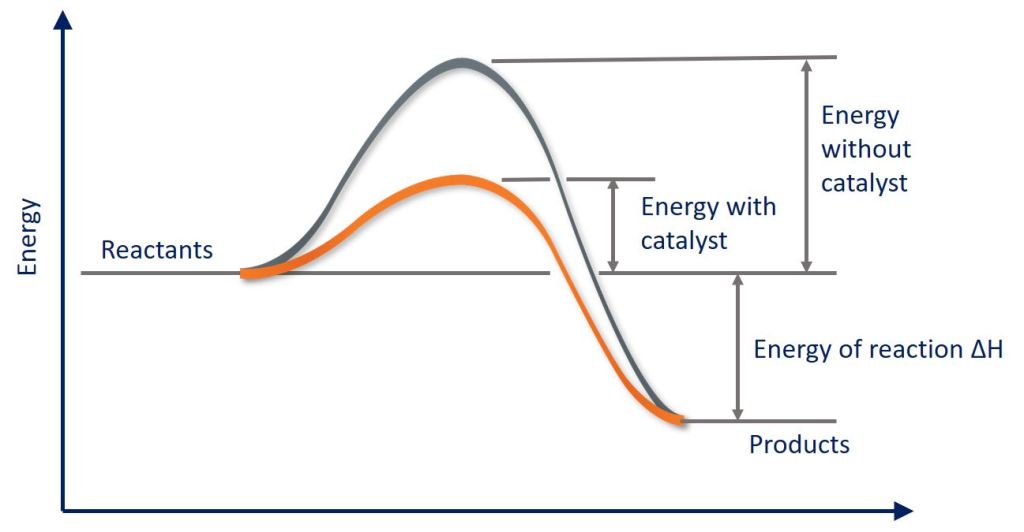
Catalyst – energy booster
A catalyst is a substance that speeds up a chemical reaction without being used up.
What is atomic simulation?
It is a computational method that models and predicts the behavior of materials and systems at the atomic level by simulating interactions between individual atoms.
We simulate catalysts, calculate reaction energies, and identify the best candidates for upcycling waste into useful products.
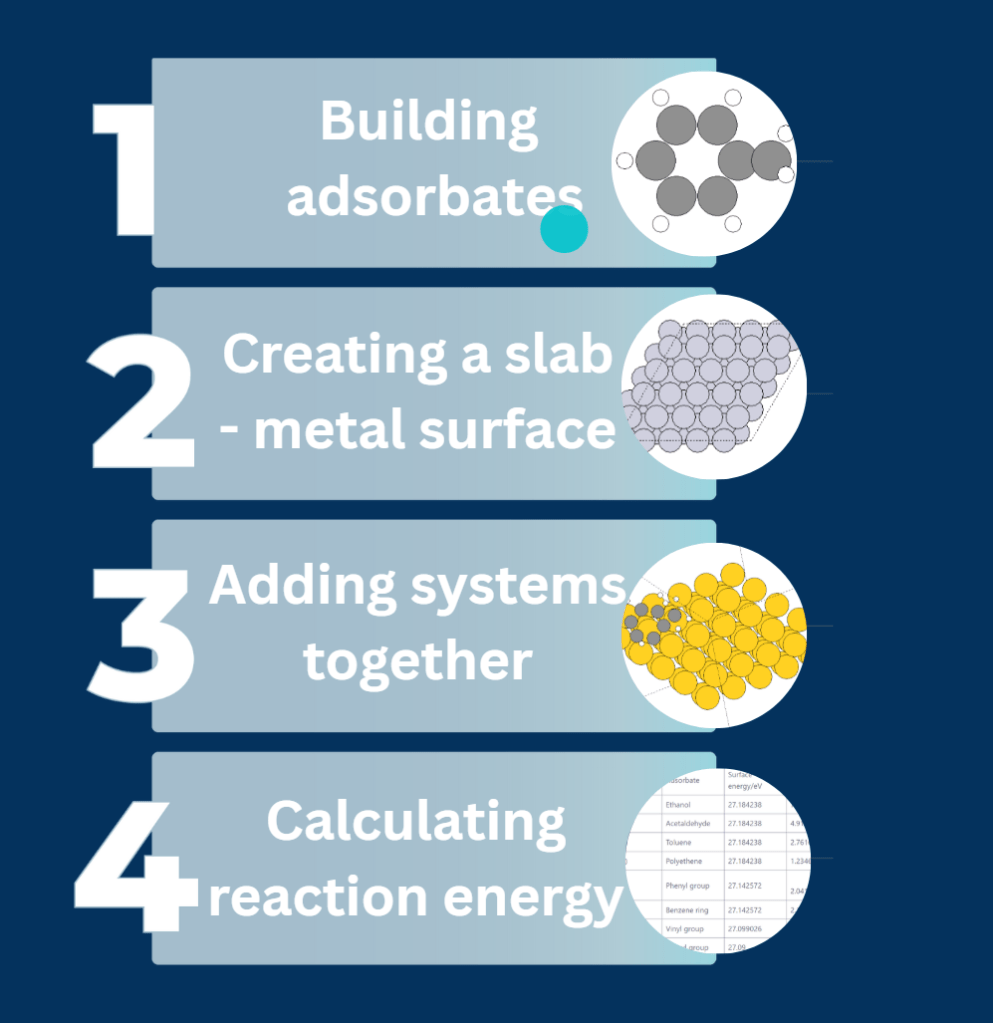
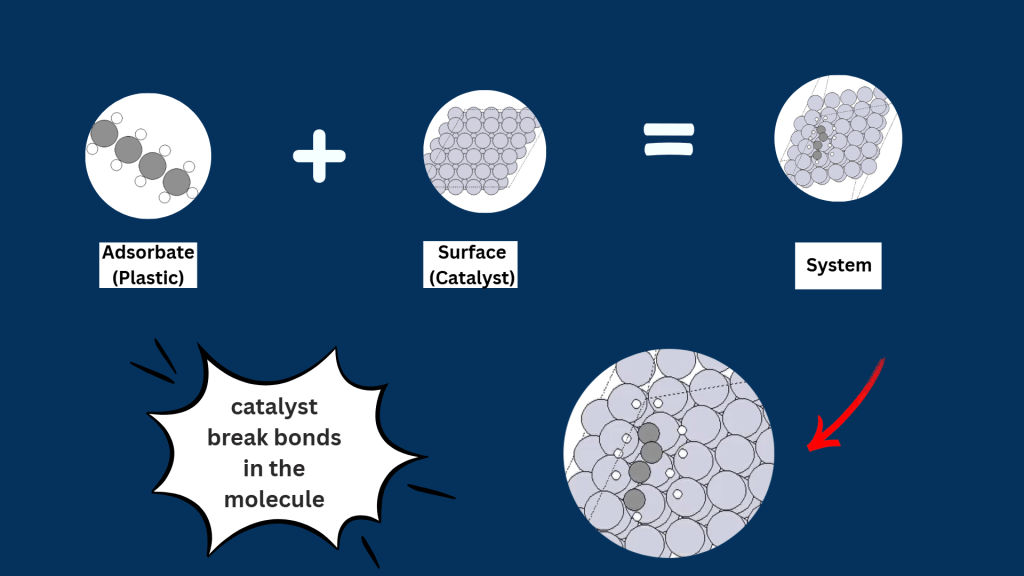
What did we simulate?
Throughout the project, we as a team did
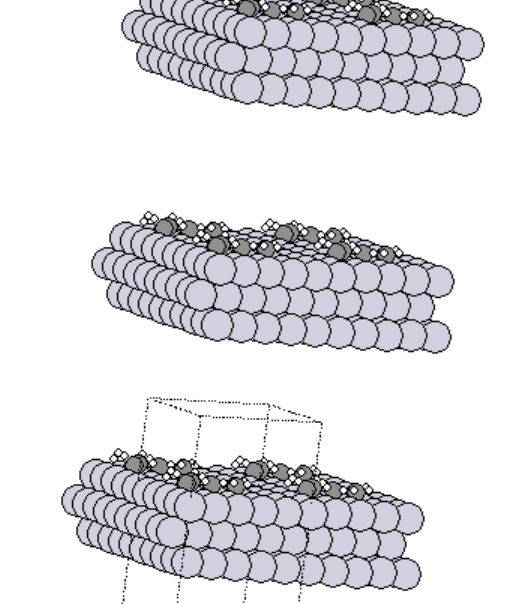
- Basic simulation using the “Read” module to simulate transitions metals and simple molecules taken from Materials Project
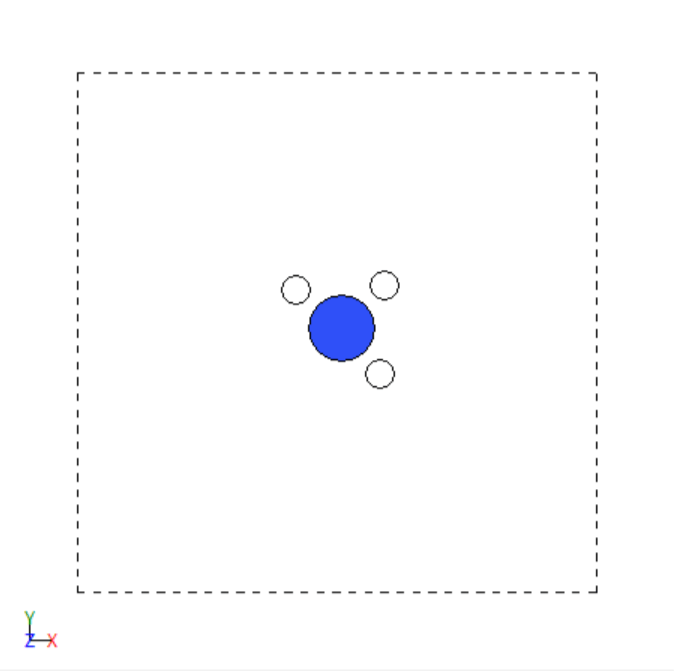
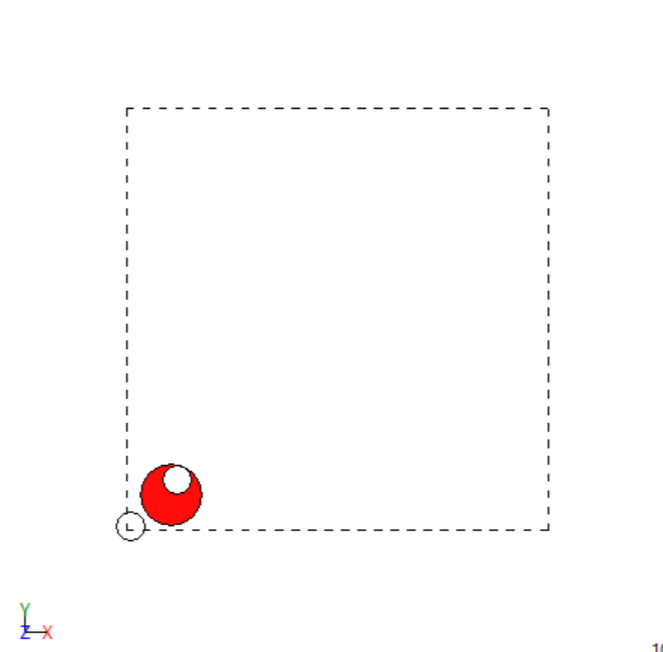
- Trial and error simulations with BFGS optimization to find more stable configuration
Before optimization
After optimization
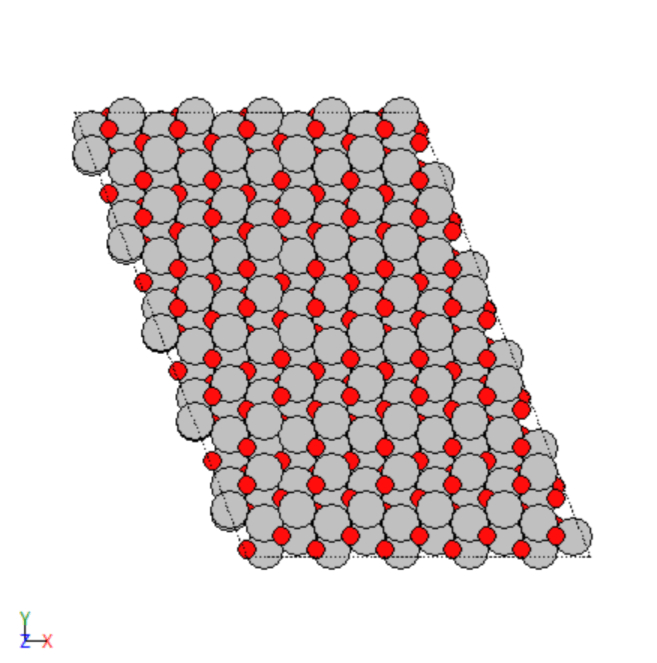
- Created 2D and 3D images of various transition metals.
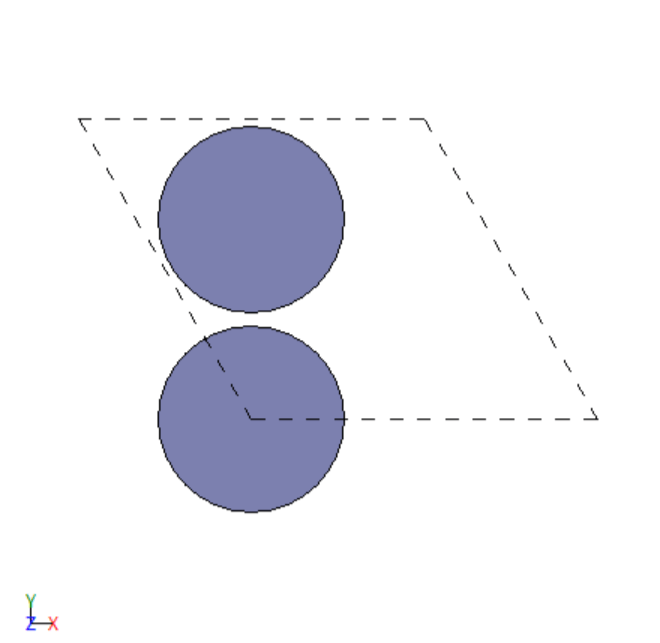
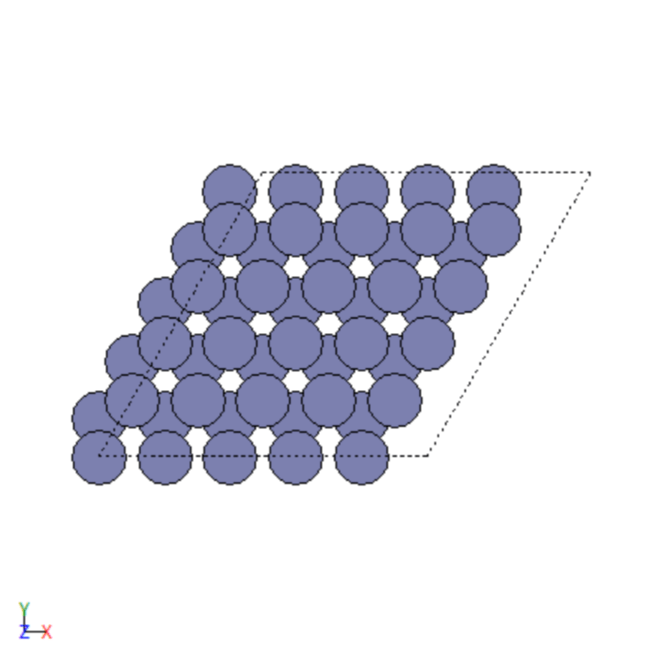
- Repeated trial and error simulations to model different adsorbates.
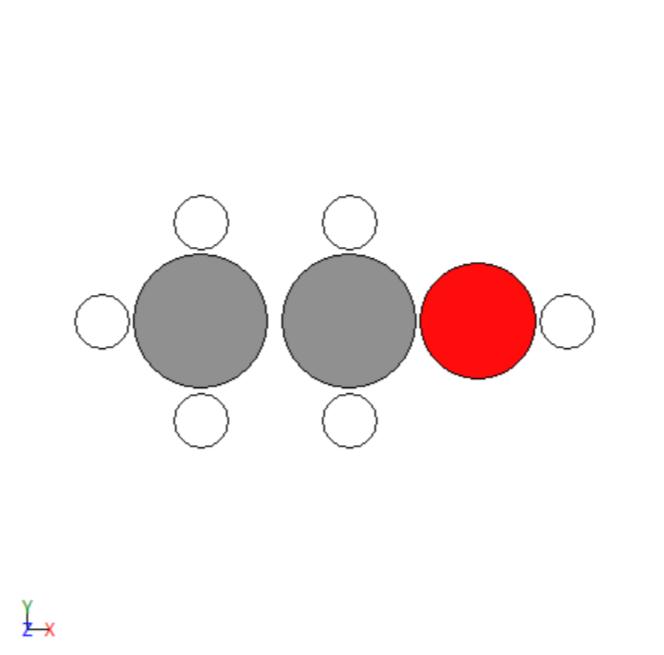
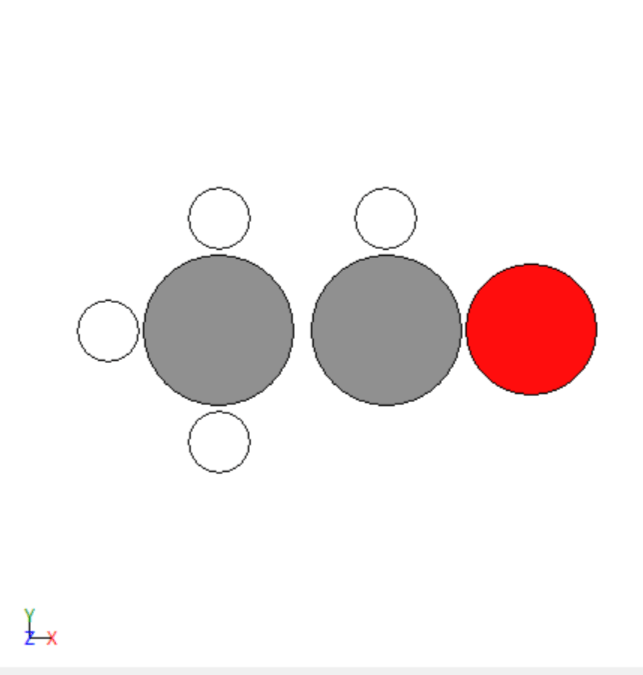
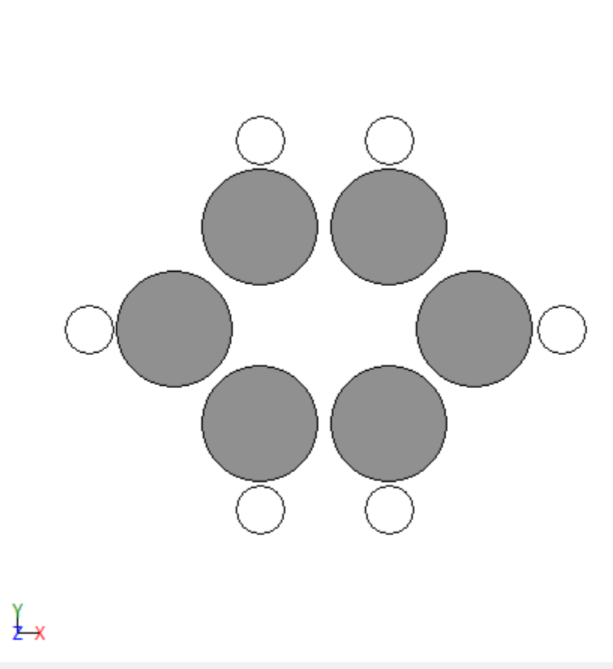
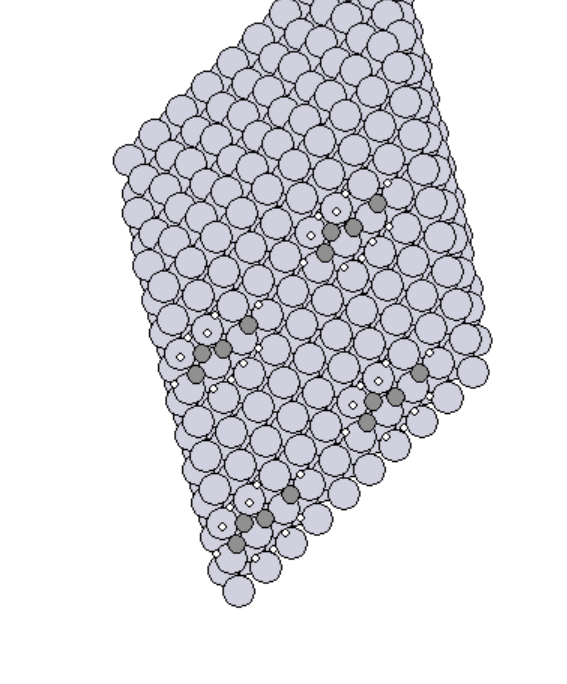
- Simulated the adsorption of various adsorbates on the transition metals surfaces.
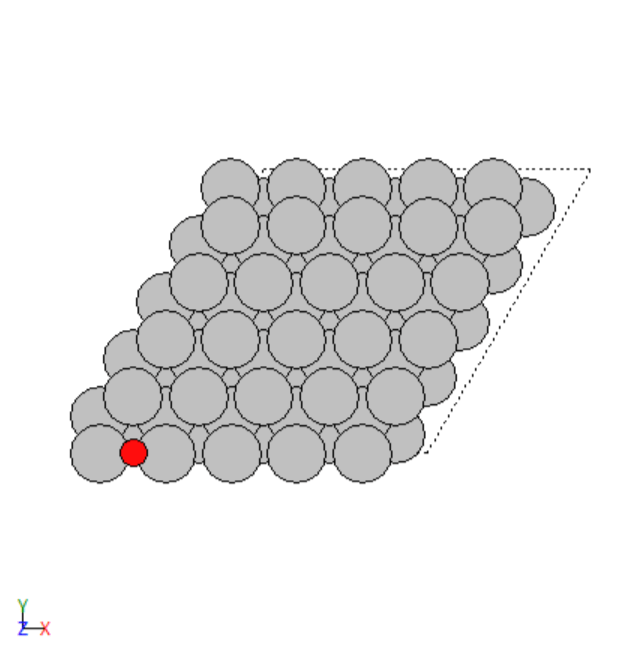
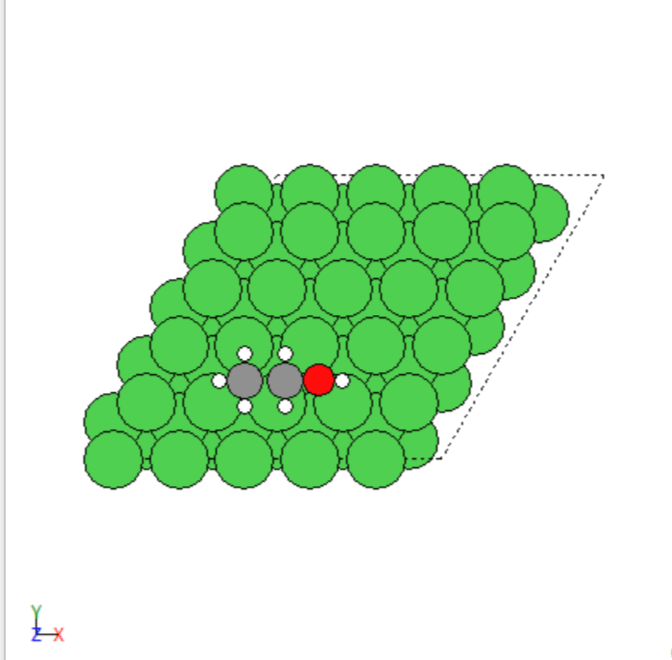
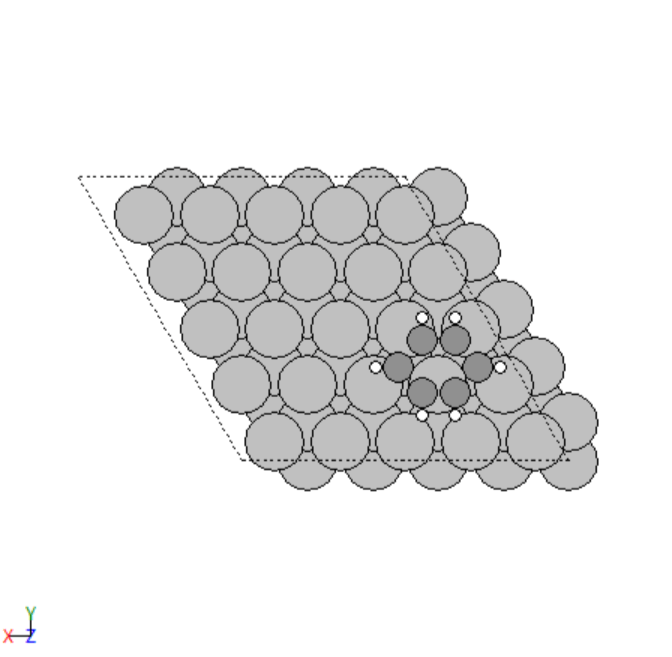
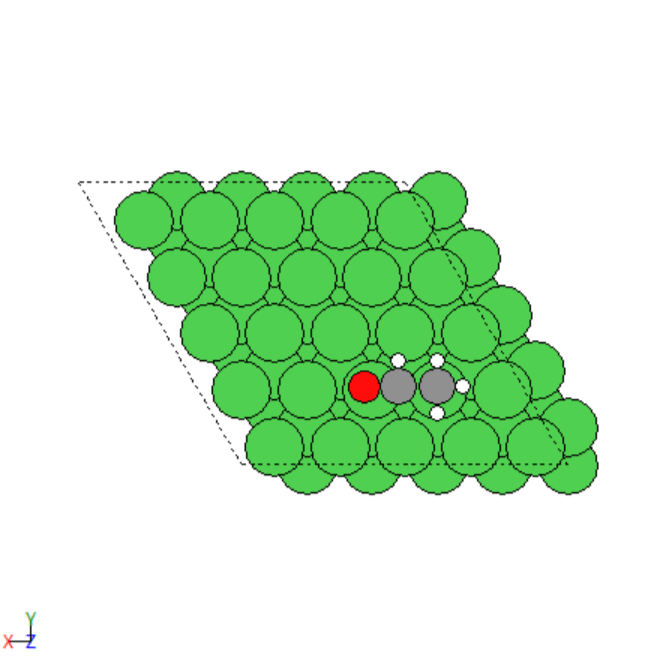
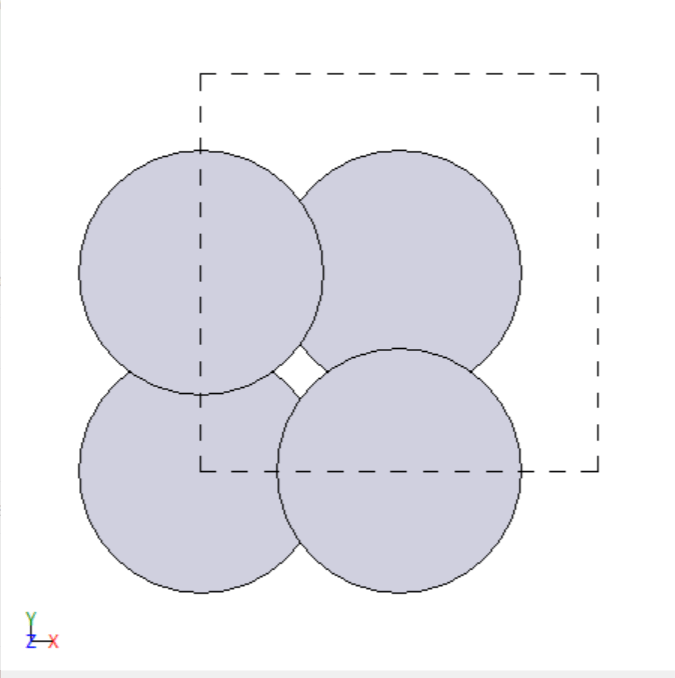
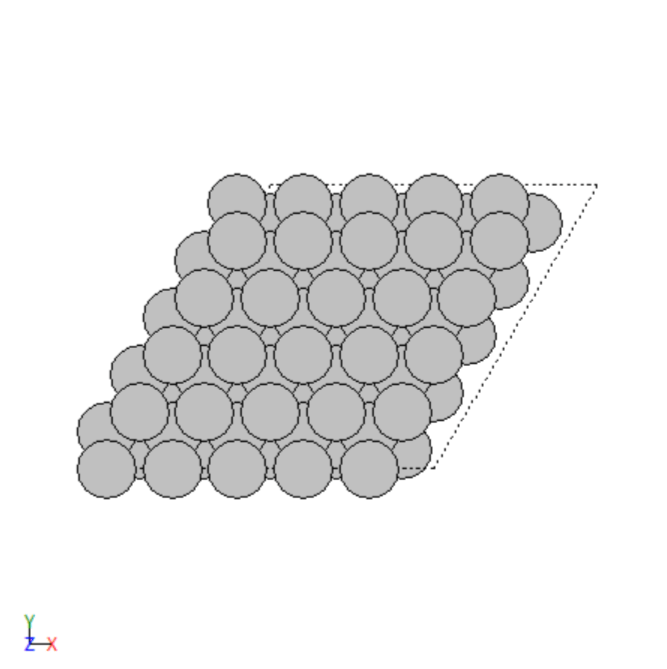
- Manually created 2D surface models of metal oxides
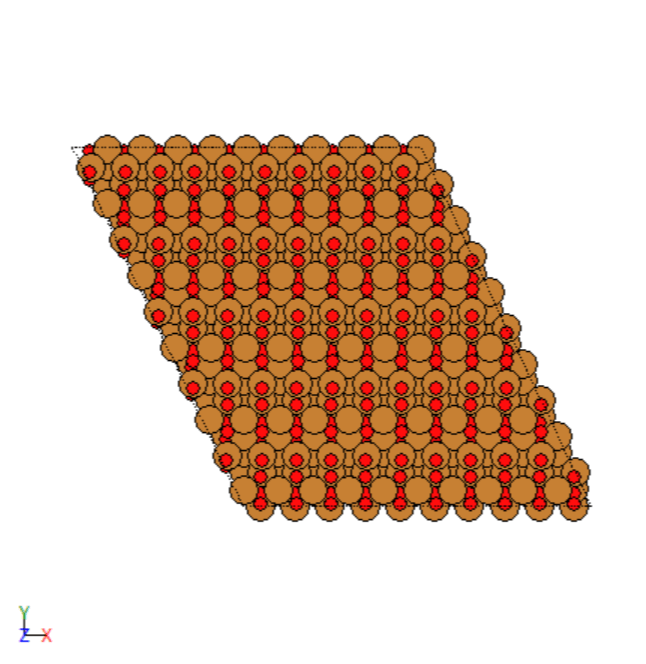
2D surface
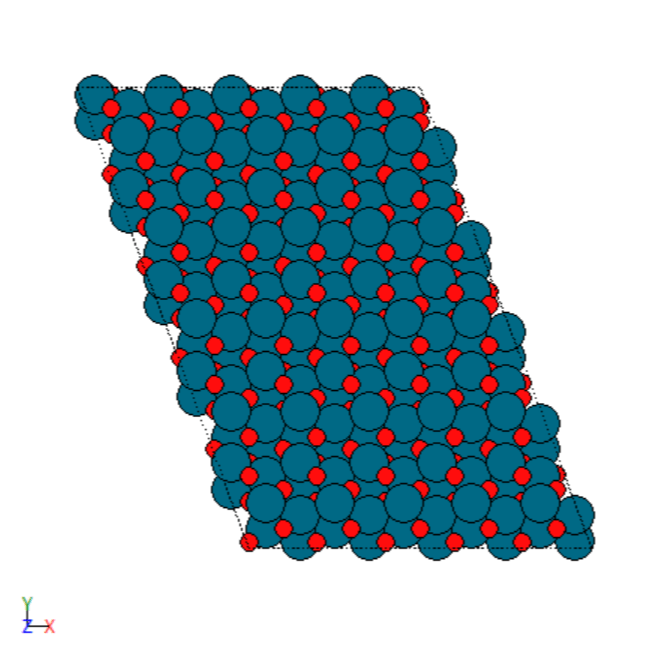
2D surface